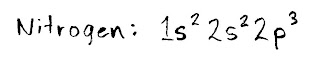(Remember to include every orbital that you cross, on the way to get to the element!)
1) Zinc
2) Sulfur
3) Bromine
4) Cadmium
For example, let's do nitrogen. You'll need the HALF SHEET elements table for this, as it shows you where the different orbitals (s, p, d and f) are.
Start in teh top left box (hydrogen, or "H") and count every electron that you pass as you move in a line to the right. I first pass two electrons in the 1s section, then two electrons in the 2s section, and finally three in 2p. Since we've now arrived at and counted the electron for the symbol "N," we're done. You would write the result like this: