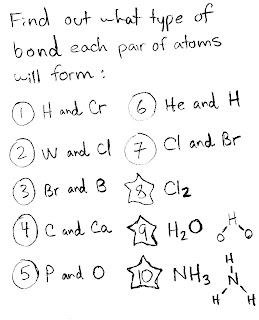
If you are or have been having trouble with either of these two concepts, then please take some time to read the two documents I discovered below:
Bonding_reading
Periodic_Table_reading
Each one is a quick read (shouldn't take more than 10-15 minutes) and it does a great job of explaining these concepts using simple language.
If you were looking for the extra credit/grade improvement assignments, read the posting below...
Saturday, December 17, 2011
TWO ways to improve your grade over winter break...
First off, let me say that I hope you are having a wonderful winter break so far. Since I knew you'd miss chemistry and/or want to improve your grade by doing more chemistry practice, I will include here the three most straightforward ways you can improve your grade:
--> #1) If your list of assignments on Gradebook Online (CPS student portal) shows you to be "missing" any of the NOTES assignments, you can still get these made up while at home. Just use Ms. Brown's digitized notes:
Part I (Aug. 9th-Sept. 7th)
Part II (Sept. 8th-Oct. 18th)
Part III (Oct. 19th-Dec.15th)
(click on the part you need to copy down notes from; and, give it a minute to load; the page will be blank at first)
Once you have handwritten notes with the same info she has into YOUR binder, I can give you the late make-up credit (which is better than a zero) for each week that you copied and got into your binder.
--> #2) Complete the extra credit packet that I have posted for winter intermission: WinterPacket
Anyone can complete the packet, even if you are missing other assignments.
!NOTE! Some of you may be aware two other extra credit projects that I created, the instructions to which have been posted in the chemistry classroom for the past couple of weeks. If you know the instructions, and are caught up with all your other missing work, then feel free to work on either of these as well.
Have fun, for sure -- but also get some work done over the long break!
Mr. Mikulski
--> #1) If your list of assignments on Gradebook Online (CPS student portal) shows you to be "missing" any of the NOTES assignments, you can still get these made up while at home. Just use Ms. Brown's digitized notes:
Part I (Aug. 9th-Sept. 7th)
Part II (Sept. 8th-Oct. 18th)
Part III (Oct. 19th-Dec.15th)
(click on the part you need to copy down notes from; and, give it a minute to load; the page will be blank at first)
Once you have handwritten notes with the same info she has into YOUR binder, I can give you the late make-up credit (which is better than a zero) for each week that you copied and got into your binder.
--> #2) Complete the extra credit packet that I have posted for winter intermission: WinterPacket
Anyone can complete the packet, even if you are missing other assignments.
!NOTE! Some of you may be aware two other extra credit projects that I created, the instructions to which have been posted in the chemistry classroom for the past couple of weeks. If you know the instructions, and are caught up with all your other missing work, then feel free to work on either of these as well.
Have fun, for sure -- but also get some work done over the long break!
Mr. Mikulski
Thursday, December 15, 2011
Friday, December 9, 2011
Thursday, December 8, 2011
Tuesday, December 6, 2011
HW#13a -- Tuesday, Dec. 6th
For HW today, you will need to complete the front and back sides of the worksheet that we started on in class. In addition to the two examples that we did (on the front side), I assigned that you write the descriptive paragraph about the following elements:
1) sodium
2) nitrogen
3) beryllium
4) bromine
5) boron
6) oxygen
Once completed, please keep this worksheet in your notes for when I do a notebook check of your Dec. 6th work.
1) sodium
2) nitrogen
3) beryllium
4) bromine
5) boron
6) oxygen
Once completed, please keep this worksheet in your notes for when I do a notebook check of your Dec. 6th work.
Monday, November 21, 2011
Becoming a father! And, how to keep working hard...
Students,
My sincerest apologies that our instruction will be interrupted for the next week or so. I need to support my wife, our new child, and her family visiting from Germany in the coming days.
You may be relieved to find that there will be no online HW for a while. :) However, please take heed of the instructions written on the whiteboard in class. I will summarize them here:
#1) The 'crystal lab' report is still due this Tuesday. Have a look at your vials, and see if you were able to grow crystals to compare to one another. If your crystals did not grow, you can look at the ones that I grew and use them for your data (you can find them on the far right end of the counter). Turn in a COMPLETE lab report (use the 'Golden Lab' guide to be sure you've done it correctly) to Ms. Payne, and make sure everyone who helped your lab group's name is on it (keeping in mind that no groups had more than four members).
#2) The work that Ms. Payne assigns you all during this 'intermission' period will be graded and entered into gradebook as 'Practice' when I return. She may choose either to collect it every day, or have you keep it in your binders. It will be a 180-point assignment, so please treat the practice seriously.
#3) If you have made up all missing work notes and tests, and want to improve your grade, you can begin working on either of the TWO extra credit assignments that are explained on the small whiteboard next to the "library" in the classroom. You can even do each project multiple times (i.e., read and report on two articles; or plan and conduct two experiments) -- as long as each report is different. Those of you still needing to take an old, 'missing' test, I will try to come after school today (Monday), so please visit my room at 3:10 to see if I am around!
Finally, I will be posting your grades on the most recent test "Ions and Ionic compounds" later today -- so check your grades online to see how you did.
if you feel like it, please either wish my wife and I luck, or keep us in your prayers.
Sincerely,
Mr. Mikulski
P.S. -- Honors students, I have graded the four NatGeo article reports that were turned in on time; please check your grades and if you received a 45%, go ahead and start on a redo (with a new article). READ THE INSTRUCTIONS FOR THE ASSIGNMENT! Those of you in honors who have not yet turned in the article (and thus have a "mi") can still do so, albeit with a late penalty. Don't turn them in to Ms. Payne; instead hold on to them until I return.
My sincerest apologies that our instruction will be interrupted for the next week or so. I need to support my wife, our new child, and her family visiting from Germany in the coming days.
You may be relieved to find that there will be no online HW for a while. :) However, please take heed of the instructions written on the whiteboard in class. I will summarize them here:
#1) The 'crystal lab' report is still due this Tuesday. Have a look at your vials, and see if you were able to grow crystals to compare to one another. If your crystals did not grow, you can look at the ones that I grew and use them for your data (you can find them on the far right end of the counter). Turn in a COMPLETE lab report (use the 'Golden Lab' guide to be sure you've done it correctly) to Ms. Payne, and make sure everyone who helped your lab group's name is on it (keeping in mind that no groups had more than four members).
#2) The work that Ms. Payne assigns you all during this 'intermission' period will be graded and entered into gradebook as 'Practice' when I return. She may choose either to collect it every day, or have you keep it in your binders. It will be a 180-point assignment, so please treat the practice seriously.
#3) If you have made up all missing work notes and tests, and want to improve your grade, you can begin working on either of the TWO extra credit assignments that are explained on the small whiteboard next to the "library" in the classroom. You can even do each project multiple times (i.e., read and report on two articles; or plan and conduct two experiments) -- as long as each report is different. Those of you still needing to take an old, 'missing' test, I will try to come after school today (Monday), so please visit my room at 3:10 to see if I am around!
Finally, I will be posting your grades on the most recent test "Ions and Ionic compounds" later today -- so check your grades online to see how you did.
if you feel like it, please either wish my wife and I luck, or keep us in your prayers.
Sincerely,
Mr. Mikulski
P.S. -- Honors students, I have graded the four NatGeo article reports that were turned in on time; please check your grades and if you received a 45%, go ahead and start on a redo (with a new article). READ THE INSTRUCTIONS FOR THE ASSIGNMENT! Those of you in honors who have not yet turned in the article (and thus have a "mi") can still do so, albeit with a late penalty. Don't turn them in to Ms. Payne; instead hold on to them until I return.
Tuesday, November 15, 2011
Monday, November 14, 2011
No HW for Monday, Nov. 14th
No new assignment for today, as we spent the day checking HW from the previous week and completing the middle part of our crystal lab.
Wednesday, November 9, 2011
Tuesday, November 8, 2011
HW for Tuesday, Nov. 8th...
Monday, November 7, 2011
Wednesday, November 2, 2011
Tuesday, November 1, 2011
Monday, October 31, 2011
Friday, October 28, 2011
HW#9c -- Thursday/Friday, Oct. 27th/28th
For the assignment below, if you do not have your 'electronegativity map' that we colored in class, you can use the link >HERE instead.


Wednesday, October 26, 2011
No HW for Wednesday, Oct. 26th
You should spend your time this evening finishing your "Moles Lab" report, as we started the lab report on Monday and it is due tomorrow (Thursday) at the end of the day.
Tuesday, October 25, 2011
HW#9b -- Tuesday, Oct. 25th
Use the link >HERE to help you find the answers to the questions below.
!!! Remember to use the analogy of butter from the fridge to the frying pan. As it heats up more and more, it goes from being a solid, to liquid, and finally to gas as parts of it are scorching. This should help you find your answers from the data table.

!!! Remember to use the analogy of butter from the fridge to the frying pan. As it heats up more and more, it goes from being a solid, to liquid, and finally to gas as parts of it are scorching. This should help you find your answers from the data table.

HW #9a -- Monday, Oct. 24th
Monday, October 24, 2011
Link to grade your extra credit HW packet
The students who turned in their extra credit HW by Tuesday End-Of-Day need to grade their work, in the same way that we grade the weekly homework. It is always important to review problems practiced at home in this way!
The correct answers are here (CLICK). Make sure that you use a marker or different color pen to mark what is correct and incorrect, and to add relevant comments whenever you can.
I know I won't be there with you to help you with additional questions you have, so do your best grading your work at home. The internet might help you write some good comments!
The correct answers are here (CLICK). Make sure that you use a marker or different color pen to mark what is correct and incorrect, and to add relevant comments whenever you can.
I know I won't be there with you to help you with additional questions you have, so do your best grading your work at home. The internet might help you write some good comments!
Friday, October 21, 2011
No HW for Friday, Oct. 21
We took an interim for the entire period today. I am also being nice and easing you all back into HW for our first week back. We will have at least THREE assignments next week, so don't expect every week to be like this!
If you did not finish your interim test -- meaning, fill in ALL bubbles, and make an educated guess on every problem -- then you MUST come in during lunch on Monday to do so. I have to scan them Monday evening, and without anwering all questions, you will get a failing grade.
If you did not finish your interim test -- meaning, fill in ALL bubbles, and make an educated guess on every problem -- then you MUST come in during lunch on Monday to do so. I have to scan them Monday evening, and without anwering all questions, you will get a failing grade.
Thursday, October 20, 2011
No new HW for Thursday, Oct. 20th
Tomorrow you will be taking an interim test to see what your strengths and weaknesses are with specific science reading and data interpretation skills. Do yourself a favor -- get some sleep and get to school on time and in good spirits tomorrow! You can relax (at least a little)once the weekend comes :)
Those of you who turned in your extra credit HW packet, you will be getting those back tomorrow as well, so that you can take them home and grade them (mark which ones you got right, which ones you got wrong, and why). You'll need to visit the Oct. 19th entry to find the key for it.
Best,
Mr. Mikulski
Those of you who turned in your extra credit HW packet, you will be getting those back tomorrow as well, so that you can take them home and grade them (mark which ones you got right, which ones you got wrong, and why). You'll need to visit the Oct. 19th entry to find the key for it.
Best,
Mr. Mikulski
Wednesday, October 19, 2011
Link to grade your extra credit HW packet
The students who turned in their extra credit HW by Tuesday End-Of-Day need to grade their work, in the same way that we grade the weekly homework. It is always important to review problems practiced at home in this way!
The correct answers are here (CLICK). Make sure that you use a marker or different color pen to mark what is correct and incorrect, and to add relevant comments whenever you can.
I know I won't be there with you to help you with additional questions you have, so do your best grading your work at home. The internet might help you write some good comments!
The correct answers are here (CLICK). Make sure that you use a marker or different color pen to mark what is correct and incorrect, and to add relevant comments whenever you can.
I know I won't be there with you to help you with additional questions you have, so do your best grading your work at home. The internet might help you write some good comments!
Tuesday, October 18, 2011
Monday, October 17, 2011
No HW Monday, Oct. 17th...
I did not post an assignment on Monday because we need to learn how to calculate the molar mass of compounds first. We got to the first part of it, but won't get to the second part until Tuesday.
You can expect to have a larger assignment posted tomorrow (Tues.) that lets us all practice doing gram <--> mole conversions outside of class.
best,
Mr. Mikulski
You can expect to have a larger assignment posted tomorrow (Tues.) that lets us all practice doing gram <--> mole conversions outside of class.
best,
Mr. Mikulski
Thursday, September 29, 2011
No new HW assignment for Thursday, Sept. 29
We took the atoms test today. You will be able to see how you did over the next few days if you check your gradebook online account at student.cps.k12.il.us
Otherwise, the grades will be posted, along with your most recent binder and HW grades, on the wall when you return.
Those of you failing due to a lack of completed work on time, please be smart and use the time over the break to do any one of three things to improve your grade:
1) Complete old HW that you never did on time (this includes any HW that you wrote down in class on Monday while we were checking it, because this is NOT doing it on time)
2) If you missed any days of class, get the missing do nows, notes and worksheets from a classmate and copy them into your binder.
3) Complete the extra credit HW packet. If you did not pick it up from me on Thursday after school, you can get it here (click for link).
Have a good break, and get your mind right for school when we pick back up again on Oct. 17th.
-- Mr. Mikulski
Otherwise, the grades will be posted, along with your most recent binder and HW grades, on the wall when you return.
Those of you failing due to a lack of completed work on time, please be smart and use the time over the break to do any one of three things to improve your grade:
1) Complete old HW that you never did on time (this includes any HW that you wrote down in class on Monday while we were checking it, because this is NOT doing it on time)
2) If you missed any days of class, get the missing do nows, notes and worksheets from a classmate and copy them into your binder.
3) Complete the extra credit HW packet. If you did not pick it up from me on Thursday after school, you can get it here (click for link).
Have a good break, and get your mind right for school when we pick back up again on Oct. 17th.
-- Mr. Mikulski
Wednesday, September 28, 2011
Tuesday, September 27, 2011
No new HW assignment for Tuesday, Sept. 27
The classes that met today were given time to begin work on a 'Make-up HW' assignment, where you turn in all HW that you have not done on time throughout the year. Do NOT turn this assignment in one piece at a time; all of this work is to be stapled together and turned in during the first few days we are back in school (starting Oct. 17).
Please use common sense and realize that you must complete all of the assignments for a given week, in order to get the check minus makeup credit for that week.
Please use common sense and realize that you must complete all of the assignments for a given week, in order to get the check minus makeup credit for that week.
Monday, September 26, 2011
HW#7a -- Monday, Sept. 26
Write down the electron configuration for these elements:
(Remember to include every orbital that you cross, on the way to get to the element!)
1) Zinc
2) Sulfur
3) Bromine
4) Cadmium
For example, let's do nitrogen. You'll need the HALF SHEET elements table for this, as it shows you where the different orbitals (s, p, d and f) are.
Start in teh top left box (hydrogen, or "H") and count every electron that you pass as you move in a line to the right. I first pass two electrons in the 1s section, then two electrons in the 2s section, and finally three in 2p. Since we've now arrived at and counted the electron for the symbol "N," we're done. You would write the result like this:
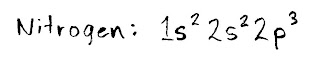
(Remember to include every orbital that you cross, on the way to get to the element!)
1) Zinc
2) Sulfur
3) Bromine
4) Cadmium
For example, let's do nitrogen. You'll need the HALF SHEET elements table for this, as it shows you where the different orbitals (s, p, d and f) are.
Start in teh top left box (hydrogen, or "H") and count every electron that you pass as you move in a line to the right. I first pass two electrons in the 1s section, then two electrons in the 2s section, and finally three in 2p. Since we've now arrived at and counted the electron for the symbol "N," we're done. You would write the result like this:
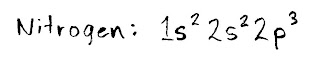
Friday, September 23, 2011
Thursday, September 22, 2011
Wednesday, September 21, 2011
HW#6c -- Wednesday, Sept. 21
For HW on this day, finish problems #1-6 on the 'Bohr model' worksheet that we started on today in class. You are welcome to try the last two or three, marked with stars, for bonus credit. Remember, there are three rules:
1) Fill in the energy levels closest to the nucleus first
2) Do not move up to the next energy level until you've filled the one before it (1st ring fills with two electrons, 2nd ring fills with eight, 3rd ring fills with eight, etc.)
3) Only use exactly as many electrons as you have. For example, Boron's atomic number is 5, meaning that it will have five protons and, being neutral, it will have exactly five electrons.
You may find this online periodic table (click here) helpful for completing this exercise, and it might also help you finish coloring, labelling and writing two facts about each of the families on your periodic table.
1) Fill in the energy levels closest to the nucleus first
2) Do not move up to the next energy level until you've filled the one before it (1st ring fills with two electrons, 2nd ring fills with eight, 3rd ring fills with eight, etc.)
3) Only use exactly as many electrons as you have. For example, Boron's atomic number is 5, meaning that it will have five protons and, being neutral, it will have exactly five electrons.
You may find this online periodic table (click here) helpful for completing this exercise, and it might also help you finish coloring, labelling and writing two facts about each of the families on your periodic table.
Tuesday, September 20, 2011
HW#6b -- Tuesday, Sept. 20
Your homework for today is to finish labelling with color the seven element families on the period table. In the end, your table should have:
Alkali Metals
Alkaline-Earth Metals
Halogens
Noble Gases
Transition Metals
Lanthanides
Actinides
You should write the name of each family on the front, next to the family. Also, you need to have two facts written down for each family (can be on the front or the back).
If you're not going to use the textbooks in our classroom to finish working on this (i.e., during lunch, after school), then you can read about the families here (click for link). Just click each different link at the bottom left to read about the different families.
Keep in mind that there are still three more families close to where the 'staircase' is on the table (by silicon, for example) that we will talk about later.
Alkali Metals
Alkaline-Earth Metals
Halogens
Noble Gases
Transition Metals
Lanthanides
Actinides
You should write the name of each family on the front, next to the family. Also, you need to have two facts written down for each family (can be on the front or the back).
If you're not going to use the textbooks in our classroom to finish working on this (i.e., during lunch, after school), then you can read about the families here (click for link). Just click each different link at the bottom left to read about the different families.
Keep in mind that there are still three more families close to where the 'staircase' is on the table (by silicon, for example) that we will talk about later.
Monday, September 19, 2011
HW#6a -- Monday, Sept. 19
Check out this site (click for link) for an online version of the periodic table that will help you complete this assignment.


Friday, September 16, 2011
HW#5d -- Friday, Sept. 16
Thursday, September 15, 2011
HW#5c -- Thursday, Sept. 15
The HW for today is to complete the 'BrainPop - Atoms' worksheet that we worked on in class today. As usual, if you were absent, you can find this worksheet on the back table, by the pencil sharpener/tissues/lotion.
!!! Use your notes from being in class, or the internet, to look up any of the answers that you are not sure of !!!
!!! Use your notes from being in class, or the internet, to look up any of the answers that you are not sure of !!!
Wednesday, September 14, 2011
Tuesday, September 13, 2011
Monday, September 12, 2011
No HW for Monday, Sept. 12
We did not learn a new concept today, and in fact took a test on a handful of topics we recently explored. On days like this, it will often happen that I will choose to not post a new assignment. Enjoy the (brief) break!
Thursday, September 8, 2011
HW#4b -- Thursday, Sept. 8th
Wednesday, September 7, 2011
Tuesday, September 6, 2011
HW#4a -- Monday, Sept. 6th
The HW assignment for today is to finish the lab reports for both the 'Hedgeapple' and 'Maple Tree Seed' lab, if you haven't done so already. The last day to turn them in and receive any credit for them is Thursday end of-day (3:05 PM)... so make sure they make their way into the 'Lab Reports' box before then!
Thursday, September 1, 2011
HW#3d -- Thursday, Sept. 1
Last HW of the week: (Two problems I pulled from another website)
1. Classify each of the following as element, compound, homogeneous mixture, or heterogeneous mixture. Only one answer applies to each.
a. silver
b. ethyl alcohol C2H5OH
c. milk
d. aluminum
e. platinum
f. table salt NaCl
g. sugar
h. maple syrup
i. seawater
j. wine
k. chocolate chip cookies
l. vanilla ice cream
m. blood
n. iced tea
o. yellow snowball
p. the sole of a tennis shoe
1. Classify each of the following as element, compound, homogeneous mixture, or heterogeneous mixture. Only one answer applies to each.
a. silver
b. ethyl alcohol C2H5OH
c. milk
d. aluminum
e. platinum
f. table salt NaCl
g. sugar
h. maple syrup
i. seawater
j. wine
k. chocolate chip cookies
l. vanilla ice cream
m. blood
n. iced tea
o. yellow snowball
p. the sole of a tennis shoe
Wednesday, August 31, 2011
Tuesday, August 30, 2011
Monday, August 29, 2011
Friday, August 26, 2011
No HW for Friday
Due to the Wildcats football game, I was unable to upload the HW in time on Friday. My blunder is your opportunity... take this chance to make sure you've done the other two assignments this week!
Thursday, August 25, 2011
Wednesday, August 24, 2011
Tuesday, August 23, 2011
HW for Monday, Tuesday !!!
I mentioned this in class, but it makes sense to put it here in the blog, too:
The HW 'assignment' for Monday (8/23) and Tuesday (8/24) of this week will be to complete the HW from last week (Wed., Thur. and Fri.) if you haven't already, and turn it in with a late penalty. The last day to turn in the HW late is Wednesday.
There will be no opportunities to turn in late HW in the future, so please be sure to complete HW#2 (Thursday and Friday of this week) before you come to class next Monday (Aug. 29).
The HW 'assignment' for Monday (8/23) and Tuesday (8/24) of this week will be to complete the HW from last week (Wed., Thur. and Fri.) if you haven't already, and turn it in with a late penalty. The last day to turn in the HW late is Wednesday.
There will be no opportunities to turn in late HW in the future, so please be sure to complete HW#2 (Thursday and Friday of this week) before you come to class next Monday (Aug. 29).
Friday, August 19, 2011
Subscribe to:
Posts (Atom)