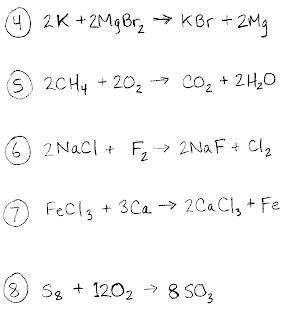Today in class, after the test, I explained an assignment where students (that means you!) go through all their notes and create at least four multiple choice problems. I asked that you divide the back of a notes sheet into four boxes, so that you have a grid in the background and so that each problem won't be too complicated or big.
Pick problems that you know you can do, on material that you enjoyed, or that you think might be a challenge to your classmates. Don't stop at just four; you can submit two or three pages (8-12 problems). Each problem of yours that I select to be on the final will win you extra credit applied toward your final exam grade.
If you have any questions, first ask your classmates; then, if they can't answer your questions, give me a call at the number provided on your syllabus.
You must have the problems completed and ready to turn in on Monday at the start of class -- so treat it like any other HW assignment.
Friday, April 27, 2012
Wednesday, April 25, 2012
Practice for test tomorrow...
Here are a few practice problems for the test tomorrow; the answers can be found by clicking on the link at the end of this post:
Use this TABLE to answer the questions:
1) What state of matter is lead in at 320 degrees C? What state of matter would it be in if its temperature increased by 250 degrees C?
2) What state of matter is manganese in at 1300 degrees C? Would its state change if its temperature decreased by 400 degrees?
3) Oxygen at -160 (negative 160) degrees Celsius is in what state? How about if it got 40 degrees C colder?
Use the four equations we've studied in class to answer the last two questions:
4) The specific heat of a substance is 1.45. If a 200-gram sample of the substance starts out at 12 degrees C and then gains 3000 J of energy, what temperature will it end up at?
5) An unknown mass of lead (Cg = 0.13) loses 1500 J of energy, and in the process its temperature goes down to 15 degrees C from 35 degrees C. What is the mass of this sample?
Once you've tried all five out, click HERE to check your work.
Use this TABLE to answer the questions:
1) What state of matter is lead in at 320 degrees C? What state of matter would it be in if its temperature increased by 250 degrees C?
2) What state of matter is manganese in at 1300 degrees C? Would its state change if its temperature decreased by 400 degrees?
3) Oxygen at -160 (negative 160) degrees Celsius is in what state? How about if it got 40 degrees C colder?
Use the four equations we've studied in class to answer the last two questions:
4) The specific heat of a substance is 1.45. If a 200-gram sample of the substance starts out at 12 degrees C and then gains 3000 J of energy, what temperature will it end up at?
5) An unknown mass of lead (Cg = 0.13) loses 1500 J of energy, and in the process its temperature goes down to 15 degrees C from 35 degrees C. What is the mass of this sample?
Once you've tried all five out, click HERE to check your work.
Monday, April 23, 2012
HW#25 -- Finish specific heat problems sheet!!!
The specific heat problems sheet was supposed to be due today, but because I didn't give good enough notice that it was the HW assignment, I gave time in class today to finish it. I won't be seeing you guys again until Thursday due to the juniors testing, so you will need to bring that worksheet in COMPLETED on Thursday so we can grade it. Remember that the second half of Thursday's lesson will be the test on solid-liquid-gas transitions and on specific heat problems.
Also, today I gave a one-day extension on the "substance adventure" story. Basically, you have all of this evening and tonight to write a better paper and email it to me. If you were already happy with the paper you had sent me last week, then you don't need to send me an edited version; I'll simply grade the one I already have from you. Any paper not in my inbox tomorrow morning when I get up (aka, about 6 AM) will not be graded and you will receive a "missing" grade for it.
If you want more details about the "substance adventure" writing assignment, read back a few posts for my example story and the instructions...
Also, today I gave a one-day extension on the "substance adventure" story. Basically, you have all of this evening and tonight to write a better paper and email it to me. If you were already happy with the paper you had sent me last week, then you don't need to send me an edited version; I'll simply grade the one I already have from you. Any paper not in my inbox tomorrow morning when I get up (aka, about 6 AM) will not be graded and you will receive a "missing" grade for it.
If you want more details about the "substance adventure" writing assignment, read back a few posts for my example story and the instructions...
Friday, April 20, 2012
HW for this week... finish problem sheet!
There is a worksheet with ten specific heat capacity' related problems on it that you received in class. If you somehow didn't ge tit, you can find it [HERE]. We've worked on it in class one day and most of you have had a second day to work on it. Since I have not assigned any other homework this week, I am assigning the completion of these ten problems as HW.
As usual, we will check the worksheet as homework (meaning, we'll go over the solutions and you'll have the opportunity to grade your work) on Monday. Recall that the four equations were:
Energy: q = m * Cg * (Tf - Ti)
mass: m = q / (Cg * [Tf - Ti])
specific heat capacity: Cg = q / (m * [Tf-Ti])
final temperature: Tf = q / (m * Cg) + Ti
For each of the ten problems, you will need to select the correct equation to use, and then plug in all the known quantities to solve the problems. Be sure when you are getting your final answer, to simplify what is inside parentheses and what is at the bottom of a fraction FIRST, before doing other calculations.
As usual, we will check the worksheet as homework (meaning, we'll go over the solutions and you'll have the opportunity to grade your work) on Monday. Recall that the four equations were:
Energy: q = m * Cg * (Tf - Ti)
mass: m = q / (Cg * [Tf - Ti])
specific heat capacity: Cg = q / (m * [Tf-Ti])
final temperature: Tf = q / (m * Cg) + Ti
For each of the ten problems, you will need to select the correct equation to use, and then plug in all the known quantities to solve the problems. Be sure when you are getting your final answer, to simplify what is inside parentheses and what is at the bottom of a fraction FIRST, before doing other calculations.
Thursday, April 12, 2012
HW#24c -- Friday, April 13th
The assignment for Friday is to finish your "Substance Adventure" essay and email it to me at sciencekidsveggies@yahoo.com
This is only if you have not already sent me the completed essay. If you send it to me completed before midnight on Friday, you can still get the maximum credit for it. If I receive it instead sometime on Saturday, it will have a late penalty, and if I receive it before next Monday at midnight, it will have an extra late penalty. After Monday, it will be too late to send it in.
These can help you finish it:
INSTRUCTIONS
SAMPLE ESSAY
This is only if you have not already sent me the completed essay. If you send it to me completed before midnight on Friday, you can still get the maximum credit for it. If I receive it instead sometime on Saturday, it will have a late penalty, and if I receive it before next Monday at midnight, it will have an extra late penalty. After Monday, it will be too late to send it in.
These can help you finish it:
INSTRUCTIONS
SAMPLE ESSAY
HW#24b -- Thursday, April 12th
The HW assignment for today is to read the page of text [HERE] and then write out and answer these questions:
1) According to the website, what are the five known states of matter (we only talked about the most common three in class)
2) As you DECREASE energy, how do the states of matter change? (ie, from what, to what next, to what next, etc.)
3) How does a chemical change differ from a physical change in a substance?
1) According to the website, what are the five known states of matter (we only talked about the most common three in class)
2) As you DECREASE energy, how do the states of matter change? (ie, from what, to what next, to what next, etc.)
3) How does a chemical change differ from a physical change in a substance?
Finishing stories that were left on a laptop... (instead of emailed)
Click [HERE] to browse for and find the file you left on a laptop yesterday.
Tuesday, April 10, 2012
HW#24a -- Tuesday, April 10th
Welcome back from spring break. This is the first of the assignments for this week's homework. Remember, these are about 20% of your grade, so it is important to take the time to practice the stuff we introduce in class at home.
For each problem below, use the table [HERE] to figure out what state (gas, liquid or solid) the sample started in, and what state it ended up in.
1) A sample of mercury starts out at 560 degrees K, and then cools down (ie, the temperature decreases) by 100 K.
2) A sample of ethyl alcohol starts out at 153 degrees K, and then it heats up (ie, the temperature increases) by 100K).
3) A sample of gold starts out at 300 degrees K (fridge temperature). Then, a chemist increases the temeprature by 1000 degrees K.
4) A sample of hydrogen starts out at 90 degrees K (colder than any temperature on earth), and then cools down even more to end up at 50 degrees K.
5) A sample of iron is in the earth's mantle (under the crust) where it is very hot -- 2,400 K. When a volcano spits out the sample onto Earth's surface, it cools down by 500 K.
6) A sample of oxygen starts out at 100 degrees K, and then its temperature is decreased to 60 degrees K.
7) A sample of nitrogen starts out at 100 degrees K, and then it is chilled down to 50 degrees K.
For each problem below, use the table [HERE] to figure out what state (gas, liquid or solid) the sample started in, and what state it ended up in.
1) A sample of mercury starts out at 560 degrees K, and then cools down (ie, the temperature decreases) by 100 K.
2) A sample of ethyl alcohol starts out at 153 degrees K, and then it heats up (ie, the temperature increases) by 100K).
3) A sample of gold starts out at 300 degrees K (fridge temperature). Then, a chemist increases the temeprature by 1000 degrees K.
4) A sample of hydrogen starts out at 90 degrees K (colder than any temperature on earth), and then cools down even more to end up at 50 degrees K.
5) A sample of iron is in the earth's mantle (under the crust) where it is very hot -- 2,400 K. When a volcano spits out the sample onto Earth's surface, it cools down by 500 K.
6) A sample of oxygen starts out at 100 degrees K, and then its temperature is decreased to 60 degrees K.
7) A sample of nitrogen starts out at 100 degrees K, and then it is chilled down to 50 degrees K.
Makeup HW assignment due Thursday, April 12th
If you were working on the makeup homework allowance I had set aside for during spring break, the last day to turn it in to the box in my room is this Thursday.
Friday, March 23, 2012
Make-up HW Project (something many of you can do over break)
I am permitting any student that has missing HW since the start of semester to do these assignments NOW, late, for 1/2 credit. This is better than the zero credit that you would otherwise get, and would help bring many of you overall averages up.
Their are a total eight assignments that apply in this project -- starting with HW#16 on Jan. 22-29th and ending with HW#23 on Mar. 11-18th. So, if you had all of them missing, and completed all eight of them and brought in your work when we come back from break, you can earn up to 5x8 or 40 points of extra practice credit to improve your grade...
You can access all previous weeks of HW by clicking on the given week in the 'Blog Archive' bar on the right side of this page
---------------------> ---------------------> ---------------------> --------------------->
If, on the other hand, you are someone that has been doing your HW, you have the possibility to earn some EXTRA practice credit:
The project is to plan, conduct, and write a lab report for your own homemade experiment. It can involve family members or friends, as long as its safe, but it should start with a question you have and a hypothesis. Give me a call using the phone number provided on the syllabus if you need help planning your experiment. Remember, every experiment has at least one INDEPENDENT VARIABLE (what the scientist is purposefully changing, or in select cases what has to "happen first" for the other variable to possibly change) and at least one DEPENDENT VARIABLE (what the scientist measures).
You can also use our lab template [HERE] as well as the 'Golden Lab Guide' [HERE] if you need help making sure your lab report is complete and of a high quality.
Their are a total eight assignments that apply in this project -- starting with HW#16 on Jan. 22-29th and ending with HW#23 on Mar. 11-18th. So, if you had all of them missing, and completed all eight of them and brought in your work when we come back from break, you can earn up to 5x8 or 40 points of extra practice credit to improve your grade...
You can access all previous weeks of HW by clicking on the given week in the 'Blog Archive' bar on the right side of this page
---------------------> ---------------------> ---------------------> --------------------->
If, on the other hand, you are someone that has been doing your HW, you have the possibility to earn some EXTRA practice credit:
The project is to plan, conduct, and write a lab report for your own homemade experiment. It can involve family members or friends, as long as its safe, but it should start with a question you have and a hypothesis. Give me a call using the phone number provided on the syllabus if you need help planning your experiment. Remember, every experiment has at least one INDEPENDENT VARIABLE (what the scientist is purposefully changing, or in select cases what has to "happen first" for the other variable to possibly change) and at least one DEPENDENT VARIABLE (what the scientist measures).
You can also use our lab template [HERE] as well as the 'Golden Lab Guide' [HERE] if you need help making sure your lab report is complete and of a high quality.
Wednesday, March 14, 2012
HW#23b -- Wednesday, March 14th
Answer each of the followijng questions, in 2-3 complete sentences. Make sure your answers are informed (use Wikipedia or other internet sources if you need to):
1) What are some organic farming practices?
2) What are some conventional farming practices?
3) How might an organic farmer respond to a bunch of insect pests coming and eating on the salad greens he's growing?
4) Why might it be good to eat food that is grown closer to where you live? Why might it be bad?
1) What are some organic farming practices?
2) What are some conventional farming practices?
3) How might an organic farmer respond to a bunch of insect pests coming and eating on the salad greens he's growing?
4) Why might it be good to eat food that is grown closer to where you live? Why might it be bad?
Monday, March 12, 2012
Thursday, March 8, 2012
HW#22b -- Friday, March 9th
For this assignment, read the following online article about plastics in the ocean [HERE] and then answer the following questions:
Before reading:
1) When you throw away garbage, where does it go? Name a few places other than the dump where you’ve seen lots of trash.
During/After reading:
2) List a few things a person could find at Kamilo Beach. List a few things a person could not find at Kamilo Beach.
3) How many tons of trash do volunteers remove from the Kamilo Beach area each year?
4) The North Pacific Ocean gyre is far from land. So how does so much plastic trash end up in the gyre?
5) Describe how scientists collect and study samples from gyres.
6) Describe the different ways that sea life interacts with the floating plastic. (Hint: How do barnacles use floating plastic? Do some animals eat the plastic?) Are these interactions good or bad?
7) Why are the smaller pieces of plastic floating at sea so dangerous?
Before reading:
1) When you throw away garbage, where does it go? Name a few places other than the dump where you’ve seen lots of trash.
During/After reading:
2) List a few things a person could find at Kamilo Beach. List a few things a person could not find at Kamilo Beach.
3) How many tons of trash do volunteers remove from the Kamilo Beach area each year?
4) The North Pacific Ocean gyre is far from land. So how does so much plastic trash end up in the gyre?
5) Describe how scientists collect and study samples from gyres.
6) Describe the different ways that sea life interacts with the floating plastic. (Hint: How do barnacles use floating plastic? Do some animals eat the plastic?) Are these interactions good or bad?
7) Why are the smaller pieces of plastic floating at sea so dangerous?
HW#22a -- Thursday, March 8th
Your HW assignment for today is to write a four-paragraph essay about rubber and plastics. The first two paragraphs should have 3-5 sentences each, and should explain what rubber is, what natural sources we get rubber from, and the things we use rubber for. The last two paragraphs should explain what plastics are, how we make them, and the things we use plastics for.
One of the best sources for this assignment is wikipedia, which is a great starting place. It will certainly give you a good definition for "rubber" and "plastics." It should also show you how we get these substances, as well as how they are used to make things around the world!
One of the best sources for this assignment is wikipedia, which is a great starting place. It will certainly give you a good definition for "rubber" and "plastics." It should also show you how we get these substances, as well as how they are used to make things around the world!
Friday, March 2, 2012
Thursday, March 1, 2012
HW#21a -- Thursday, March 1st
Sorry, I was a little lit later uploading this than I had hoped (garden club meeting went later than I expected!):


////////////////////////////////////////////////


////////////////////////////////////////////////
This assignment will be good practice for the test next week, as it has all three skills in it -- "identify reaction type," "find molar mass of chemical species involved in a reaction," and "use molar ratios to solve stoichiometry problems."
Wednesday, February 29, 2012
No HW for Wednesday -- instead, finish in-class assignment:
Thursday, February 23, 2012
No new online work today... just finish in-class practice

These were the problems that we looked at in class; actually, I believe that 1st period saw some slightly different problems. If you were in 1st period, just make sure you've worked out at least FOUR problems total as practice. Have the work on your notes sheet, and store it in your binder so you can show me during a binder check.
Wednesday, February 22, 2012
Tuesday, February 21, 2012
Friday, February 17, 2012
HW#19b -- Friday, Feb. 17th
Complete these six problems by determining which type of reaction each is. On notebook paper, write out each reaction first. Remember that each reaction MUST be one of the five types -- combustion, synthesis, decomposition, single replacement, or double replacement.
If you want extra credit (bringing your graded HW up to a check double plus), then after saying what type the reaction is, go on and balance the equation (determine what the coefficients should be).

PS -- Yes, these are the last six problems from the reaction types worksheet that was handed out in class. This puts you that much closer to finishing that other assignment!
If you want extra credit (bringing your graded HW up to a check double plus), then after saying what type the reaction is, go on and balance the equation (determine what the coefficients should be).

PS -- Yes, these are the last six problems from the reaction types worksheet that was handed out in class. This puts you that much closer to finishing that other assignment!
Thursday, February 16, 2012
No online HW today -- just finish WS from class
On Thursday, you started work on a 'reaction types' worksheet in class. There is 22 problems, and you need to be able to say which type of reaction each one is. When you've finished this practice, put it back in your notebook with your Feb. 16 'DO NOW' -- and know that I will very likely be checking this day as part of the notebook check next week.
Tuesday, February 14, 2012
HW#19a -- Tuesday, Feb. 14th
See the assignment in the image below. Don't forget, it can only be one of the five types we discussed in class: combustion, synthesis, decomposition, single replacement, or double replacement. Also, remember how 'displacement' can be said in place of 'replacement' (either is acceptable).
BONUS: See if you can recognize which one is the special 'acid-base' variety of double replacement...

BONUS: See if you can recognize which one is the special 'acid-base' variety of double replacement...

Thursday, February 9, 2012
HW#18a -- Thursday, Feb. 9th
A polite reminder -- many students have not yet turned in the bottom of their Syllabus Update (golden sheet), signed by a parent or guardian. This is one of the first grades of the second semester, and an easy one at that. Don't delay in getting it done.
A second polite reminder -- since we missed binder checks the other day, I will instead do it on Friday (tomorrow), before the test. The days I will most likely check are Jan. 31 (with the Chembalancer practice on the back side) and Feb. 1 (with the Lantern Lab report as the practice).
And now, on to the HW assignment:

A second polite reminder -- since we missed binder checks the other day, I will instead do it on Friday (tomorrow), before the test. The days I will most likely check are Jan. 31 (with the Chembalancer practice on the back side) and Feb. 1 (with the Lantern Lab report as the practice).
And now, on to the HW assignment:

Wednesday, February 8, 2012
HW for today -- finish "Balancing Equations" practice WS
The HW for tonight will be to complete a piece of practice that you should keep in your notebook. This worksheet was handed out today, and was called "Balancing Equations." It has a FRONT and BACK side, with a total of six problems. For each problem, you need to complete the final count on the atoms table AND write the correct coefficients into the equations. For example, #2 should have all of this information filled in:


Wednesday, February 1, 2012
Tuesday, January 31, 2012
Tuesday, Jan. 31
No online HW assignment today; instead, make sure that you finished the 'Chembalancer' worksheet that we've worked on for the past two days in class. If you need to go back to the Chembalancer program so that you can finish the problems, you can click [HERE]
Don't forget that #12 and #13, which you need to draw out yourself, are also part of the worksheet.
Don't forget that #12 and #13, which you need to draw out yourself, are also part of the worksheet.
Monday, January 30, 2012
Thursday, January 26, 2012
Help finishing your Venn diagram (if you need it)
Here are the links to the online interactive activities that we explored in class:
Ionic Bonding
Covalent Bonding
Use them to finish your Venn diagram (four statements in each of the three sections) if you need to.
Ionic Bonding
Covalent Bonding
Use them to finish your Venn diagram (four statements in each of the three sections) if you need to.
HW#16b -- Friday, Jan. 26
Wednesday, January 25, 2012
HW for Wednesday, Jan. 25
The assignment today is to finish the lab report that we started in class. You had time enough in class to come up with your hypothesis, write down your materials and procedure, and collect your data. It is up to you to complete the last two sections -- the 'Conclusion' and the 'Evaluation' -- before Wednesday of next week. If you need to remember what belongs in these sections, use the 'Golden lab Guide' that you picked up in class a few months ago.
Have the work in your binder under the appropriate day so you can show me during the binder check!
Have the work in your binder under the appropriate day so you can show me during the binder check!
Monday, January 23, 2012
Thursday, January 19, 2012
Honors Chem HW assignment for Thursday, Jan. 19th
This is an honors-only assignment; meaning, you do not have to complete it if you are in the regular chemistry classes (1st, 5/6th, 7th, 8th):
1) Read an article in a scientific magazine or science book (3-5 pages) and, after reading the text, identify at least eight words that you did not know the definition for.
2) Write these words down, and then look up and write down their proper definition. NO one-word 'summary'-style definitions; it should be clear that you know the part of speech of the word (ie, verb, noun, adjective) and what it means.
3) Use the word in a sentence of your own making.
1) Read an article in a scientific magazine or science book (3-5 pages) and, after reading the text, identify at least eight words that you did not know the definition for.
2) Write these words down, and then look up and write down their proper definition. NO one-word 'summary'-style definitions; it should be clear that you know the part of speech of the word (ie, verb, noun, adjective) and what it means.
3) Use the word in a sentence of your own making.
Tuesday, January 17, 2012
HW for this week -- Scientific Method packet
Your HW for this week, which we started on in class on Tuesday, will be due when you come in to class next Monday (Jan. 23). There is no electronic assignments this week, only that you complete, with correct answers, the number of exercises in the packet to give you the grade that you want:
180 pts correct = check (85%)
240 pts correct = checkPlus (95%)
300 pts correct = checkDoublePlus (105%)
As is usual for HW, we will grade your work on the packet together, in class, on Monday. Good luck with your interim tests this week!
-- Mr. Mikulski
180 pts correct = check (85%)
240 pts correct = checkPlus (95%)
300 pts correct = checkDoublePlus (105%)
As is usual for HW, we will grade your work on the packet together, in class, on Monday. Good luck with your interim tests this week!
-- Mr. Mikulski
Thursday, January 12, 2012
HW#15c -- Thursday, Jan. 11th
For each experiment, first list the independent and dependent variables. Then, continue the story by describing an experiment that the person could do. In your description, show that the person got data that ended up supporting their original hypothesis. Use complete sentences!
Ex. -- 'Bowling experiment'
Mr. Kitchen thinks that if he eats a large order of fries before he bowls, that
his score will improve. He believes this because he often feels like his hunger
is distracting him.
Mr. Mikulski writes, as his answer to this question:
"INDEP: eating fries or not eating fries
DEP: score per game
Mr. Kitchens alternates either eating fries or not eating fries before his
bowling games, for the next two months. In the 49 games he bowled without the
fries, his average score was a 148. In the 50 games he bowled with fries, his
average was a 164."
------------------------------------
1) Mr. Mikulski believes that if he makes his students keep a good notebook and check online for their assignments, that more of them will be able to succeed (ie, graduate on time from) the college that they later attend.
2) Alan (a student) is pretty sure that the worse a student's attendance is, the worse their grades will be. He surveyed five students in a small experiment.
3) The security guard thinks that if he starts asking students to tuck in their uniforms nicely ("Could you please tuck your shirt in?") instead of sternly ("Tuck in your shirt, man! You know better!"), that the number of students with untucked shirts will increase.
4) Mr. Horton believes that his weight will decrease if the teachers start getting a weekly basketball game going.
5) Janelle thinks that the Chicago police P.O.D.S. (the flashing blue cameras) make the amount of on-the-street crime that happens further down the block increase.
Ex. -- 'Bowling experiment'
Mr. Kitchen thinks that if he eats a large order of fries before he bowls, that
his score will improve. He believes this because he often feels like his hunger
is distracting him.
Mr. Mikulski writes, as his answer to this question:
"INDEP: eating fries or not eating fries
DEP: score per game
Mr. Kitchens alternates either eating fries or not eating fries before his
bowling games, for the next two months. In the 49 games he bowled without the
fries, his average score was a 148. In the 50 games he bowled with fries, his
average was a 164."
------------------------------------
1) Mr. Mikulski believes that if he makes his students keep a good notebook and check online for their assignments, that more of them will be able to succeed (ie, graduate on time from) the college that they later attend.
2) Alan (a student) is pretty sure that the worse a student's attendance is, the worse their grades will be. He surveyed five students in a small experiment.
3) The security guard thinks that if he starts asking students to tuck in their uniforms nicely ("Could you please tuck your shirt in?") instead of sternly ("Tuck in your shirt, man! You know better!"), that the number of students with untucked shirts will increase.
4) Mr. Horton believes that his weight will decrease if the teachers start getting a weekly basketball game going.
5) Janelle thinks that the Chicago police P.O.D.S. (the flashing blue cameras) make the amount of on-the-street crime that happens further down the block increase.
Wednesday, January 11, 2012
Tuesday, January 10, 2012
HW#15a -- Tuesday, Jan. 10th
For each story, give five answers:
I) What is the question?
II) What is the person's hypothesis?
III) What is the independent variable?
IV) What is the dependent variable?
V) Was the hypothesis supported by the data, or not?
'The vegetable story'
---------------------
Mr. Mikulski's garden club wants to expand its operations this year, and get some student athletes to commit to taking home and cooking fresh vegetables and fruits every week. Many nutrition books say that athletes will be able run, jump, etc. longer before getting tired if they eat fresh food like this. Mr. Mikulski believes that if the athletes take home and cook the fresh food for a month, they will improve their endurance (ability to keep playing).
Mr. Mikulski gave five different athletes and their families fresh food from the garden for one month. He gave another five athletes no such food (they simply ate what they usually would at home). After one month, the athletes who ate the fresh food could play for 20% longer than when they started. The athletes who ate their usual food could play for 11% longer.
'The laundry detergent story'
-----------------------------
Latricia heard that some laundry detergents have too many chemicals in them, and can cause some people's skin to break out in rashes. Sometimes she gets skin rashes on her arms and she doesn't quite know why, but it seems to happen if she wears laundry that came right out of the drier. She believes that washing with a natural detergent (which has less chemicals) will reduce the number of arm rashes that she gets.
For the first two weeks, she washes with her normal detergent, and counts the number of rashes she gets throughout the week. She gets a total of 7 rashes. For the next two weeks, she washes her clothes with the natural detergent (less chemicals). She gets a total of 8 rashes during that time.
I) What is the question?
II) What is the person's hypothesis?
III) What is the independent variable?
IV) What is the dependent variable?
V) Was the hypothesis supported by the data, or not?
'The vegetable story'
---------------------
Mr. Mikulski's garden club wants to expand its operations this year, and get some student athletes to commit to taking home and cooking fresh vegetables and fruits every week. Many nutrition books say that athletes will be able run, jump, etc. longer before getting tired if they eat fresh food like this. Mr. Mikulski believes that if the athletes take home and cook the fresh food for a month, they will improve their endurance (ability to keep playing).
Mr. Mikulski gave five different athletes and their families fresh food from the garden for one month. He gave another five athletes no such food (they simply ate what they usually would at home). After one month, the athletes who ate the fresh food could play for 20% longer than when they started. The athletes who ate their usual food could play for 11% longer.
'The laundry detergent story'
-----------------------------
Latricia heard that some laundry detergents have too many chemicals in them, and can cause some people's skin to break out in rashes. Sometimes she gets skin rashes on her arms and she doesn't quite know why, but it seems to happen if she wears laundry that came right out of the drier. She believes that washing with a natural detergent (which has less chemicals) will reduce the number of arm rashes that she gets.
For the first two weeks, she washes with her normal detergent, and counts the number of rashes she gets throughout the week. She gets a total of 7 rashes. For the next two weeks, she washes her clothes with the natural detergent (less chemicals). She gets a total of 8 rashes during that time.
Monday, January 9, 2012
Monday, Jan. 9th -- Finish WS from class!
No online HW assignment today; just finish the worksheet that we started in class (do the Homer and the Bart experiments). When you're finished, put it back in your binder with your Jan. 9th 'Do Now'/Notes sheet.
Tuesday, January 3, 2012
Video help... (for Lewis Diagrams video test RETAKE)
If you need to retake your Lewis Diagrams test, and are having trouble understanding how to move your cards around to get the right configuration, it may be worth checking out this video:
Covalent bonds means sharing electrons!
It was made by another chemistry teacher with more experience than me, and presented in a different way that you might understand better. I am putting it up as a file instead of a YouTube link, because for whatever reason CPS doesn't allow access to YouTube while at school.
!! Remember, you have two weeks after we return from winter break to retake and pass this test !!
Looking forward to seeing you all when we come back from break!
Mr. Mikulski
Covalent bonds means sharing electrons!
It was made by another chemistry teacher with more experience than me, and presented in a different way that you might understand better. I am putting it up as a file instead of a YouTube link, because for whatever reason CPS doesn't allow access to YouTube while at school.
!! Remember, you have two weeks after we return from winter break to retake and pass this test !!
Looking forward to seeing you all when we come back from break!
Mr. Mikulski
Subscribe to:
Posts (Atom)